Polycythemia vera (PV) and essential thrombocythemia (ET) patients have a higher risk of arterial and venous thrombosis than healthy individuals; thromboses are their principal cause of morbidity and mortality. We reported increased transcription of prothrombotic and inflammatory genes in granulocytes and platelets of PV and ET. There were differences in the expression of prothrombotic genes between platelets and granulocytes, suggesting that these cells have cell-specific contributions to thrombosis in PV and ET. Some of these prothrombotic genes are regulated by hypoxia inducible factors (HIFs) (PMID: 32203583). However, the molecular mechanism of thrombosis in PV and ET remains unknown.
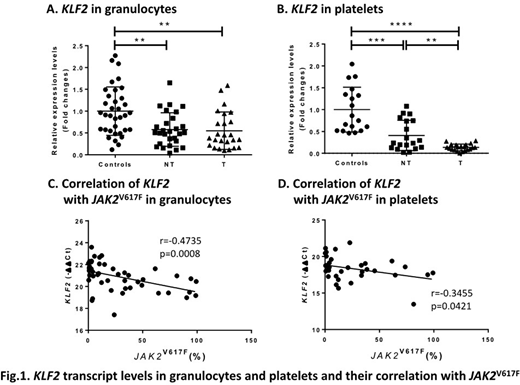
KLF2 (Kruppel like factor 2) is a transcription factor regulating primitive erythropoiesis and inflammation. Knockdown of KLF2 in cultured endothelial cells increases prothrombotic gene expression and reduces blood clotting time and flow rates (PMID: 15718498). Targeted deletion of KLF2 in neutrophils increases thrombosis by inducing the expression and activity of tissue factor (Blood, 2018, 132:75). To study the role of KLF2 in PV and ET thrombosis, we measured KLF2 mRNA in granulocytes from 53 PV and ET patients (25 with a history of thrombosis) and in platelets from 40 patients (21 with a history of thrombosis). We also measured KLF2 mRNA in granulocytes from 38 controls and platelets from 18 controls. Althrough the role of KLF2 in thrombosis has been studied in neutrophils, we also tested KLF2 mRNA in platelets since we previously observed a different pattern of expression of prothrombotic genes between granulocytes and platelets in PV and ET. We found lower KLF2 mRNA in both granulocytes and platelets of PV and ET patients compared to the controls (Fig. 1A and B). Compared to patients without thrombosis, those with thrombosis had lower KLF2 mRNA in platelets but not granulocytes. KLF2 mRNA in these cells correlated inversely with JAK2V617F allele burden in granulocytes (Fig. 1C and D). We then measured mRNA of prothrombotic genes: F3 (tissue factor), SELP (P-selectin), IRAK1 (interleukin 1 receptor associated kinase 1), IL1RAP (interleukin 1 receptor accessory protein), VEGFA (vascular endothelial growth factor-A), THSB1 (thrombospondin 1), SERPINE1 (encoding plasminogen activator inhibitor 1 [PAI-1]). The mRNA levels of these prothrombotic genes correlated inversely with KLF2 mRNA in platelets while SELP and THSB1 transcripts correlated inversely with KLF2 mRNA in granulocytes.
KLF2 and HIFs are reported to interact (PMID: 19491109, PMID: 21565532). In order to elucidate the regulatory machanism of KLF2 in thrombosis, we measured KLF2 mRNA in patients with two inherited disorders of hypoxia sensing characterized by thrombosis: 1) Chuvash erythrocytosis (CE) due to homozygous mutation of VHLR200W (13 patients) and 2) erythrocytosis due to gain-of-function mutation of HIF-2a (two patients with HIF2AM535V and two patients HIF2AE548K). KLF2 mRNA levels did not differ in granulocytes and platelets between these patients and controls. However, two CE patients and two patients with HIF2AM535V with a history of thrombosis had lower KLF2 mRNA levels compared to patients without thrombosis (ASH this meeting, 2020 Song J).
In conclusion, we report here that KLF2 transcripts are down regulated in both granulocytes and platelets from PV and ET patients and they correlate inversely with the transcripts of prothrombotic genes and JAK2V617F allelic burden, suggesting that KLF2 might be a negative regulator of thrombotic gene expression in PV and ET. Here we did not detect any changes of KLF2 transcripts in congential disorders with elevated HIFs. However, two CE patients and 2 patients with HIF2AM535V with thrombosis had less KLF2 expression compared to those without thrombosis. These results suggest that, by inference from findings in congenital disorders with elevated HIFs, KLF2 in PV and ET granulocytes and platelets may be regulated in a HIF-independent manner but that thrombosis may be regulated in a HIF-dependent manner. Thus, KLF2 may be a novel therapeutic target to prevent thrombosis in PV and ET, but confirmation by further studies is needed. The upstream regulation of KLF2 in PV and ET granulocytes and platelets needs to be elucidated.
*PT &JTP contributed equally
Gangaraju:Sanofi Genzyme, Consultant for Cold Agglutinin Disease: Consultancy. Gordeuk:CSL Behring: Consultancy, Research Funding; Global Blood Therapeutics: Consultancy, Research Funding; Imara: Research Funding; Ironwood: Research Funding; Novartis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal